Rutherford Scattering
|
|
Original Sim and Translations |
About
Topics
- Quantum Mechanics
- Atomic Nuclei
- Atomic Structure
Description
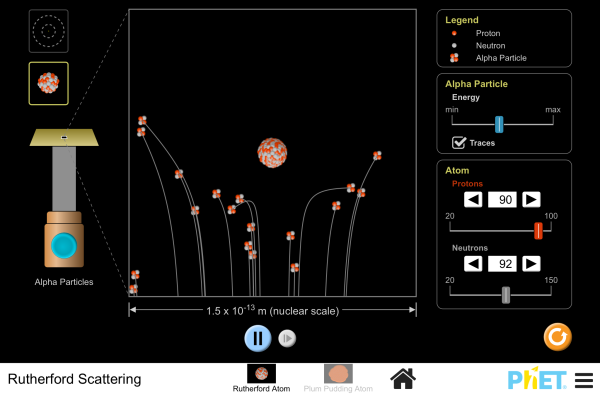
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
Sample Learning Goals
- Describe the qualitative difference between scattering off positively charged nucleus and electrically neutral plum pudding atom.
- For charged nucleus, describe qualitatively how angle of deflection depends on: Energy of incoming particle, Impact parameter, Charge of target
Keywords
For Teachers
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
| Title |
|
|
Authors | Level | Type |
|---|---|---|---|---|---|
| Atomic models homework (Inquiry based) |
|
|
Sam McKagan, Kathy Perkins and Carl Wieman | UG-Adv UG-Intro |
HW |
Browse more activities.
Translations
Related Simulations
Software Requirements
| Windows 7+ | Mac OS 10.7+ | iPad and iPad Mini with iOS | Chromebook with Chrome OS |
|---|---|---|---|
|
Internet Explorer 10+ latest versions of Chrome and Firefox
|
Safari 6.1 and up latest versions of Chrome and Firefox
|
latest version of Safari
|
latest version of Chrome
|
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|