Molarity
|
|
Original Sim and Translations |
About
Topics
- Solutions
- Molarity
- Moles
- Volume
- Solubility
- Saturation
Description
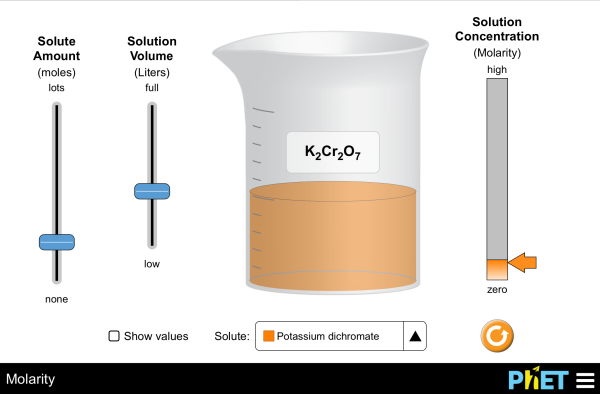
What determines the concentration of a solution? Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Change solutes to compare different chemical compounds in water.
Sample Learning Goals
- Describe the relationships between volume and amount of solute to concentration.
- Explain how solution color and concentration are related.
- Calculate the concentration of solutions in units of molarity (mol/L).
- Use molarity to calculate the dilution of solutions.
- Compare solubility limits between solutes.
Keywords
For Teachers
Teacher Tips
| Overview of sim controls, model simplifications, and insights into student thinking ( PDF ). |
Teacher-Submitted Activities
| Title |
|
|
Authors | Level | Type |
|---|---|---|---|---|---|
| Saturated Solutions - Interactive Lecture Demonstration |
|
|
Ted Clark, Julia Chamberlain | UG-Intro | Demo |
| Molarity and Dilution |
|
|
Trish Loeblein | UG-Intro HS |
Lab HW |
| Solutions Unit Sample |
|
|
Trish Loeblein | HS UG-Intro |
CQs Demo Lab HW |
| How do PhET simulations fit in my middle school program? |
|
Sarah Borenstein | MS | Other | |
| Alignment of PhET sims with NGSS |
|
Trish Loeblein | HS | Other | |
| PhET Sims Aligned to the Chemistry Curriculum |
|
Julia Chamberlain | HS UG-Intro |
Other | |
| Student Guide for PhET - Molarity in html5 | Brian Libby | MS HS |
HW Guided |
||
| MS and HS TEK to Sim Alignment | Elyse Zimmer | HS MS |
Other | ||
| Molarity Simulation | Jennifer McGehee | MS | Guided |
Browse more activities.
Translations
Related Simulations
Software Requirements
| Windows 7+ | Mac OS 10.7+ | iPad and iPad Mini with iOS | Chromebook with Chrome OS |
|---|---|---|---|
|
Internet Explorer 10+ latest versions of Chrome and Firefox
|
Safari 6.1 and up latest versions of Chrome and Firefox
|
latest version of Safari
|
latest version of Chrome
|
Credits
| Design Team | Third-party Libraries | Thanks To |
|---|---|---|
|
|